Introduction
Percutaneous coronary intervention (PCI) is a non-surgical procedure to treat many types of heart issues using thin flexible tubes (catheters), guidewires (wires that are used as a guide to place other medical devices), and stents (small structures to prop open the blood vessel).
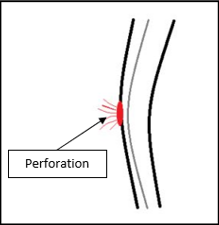 One unlikely complication of a PCI procedure is a perforation (hole) in a heart blood vessel. A perforation
can occur when a medical device such as a catheter, guidewire, or stent gets caught in the vessel wall
and pushes through the wall to create a hole that allows blood to escape the vessel. This is a rare,
life-threatening complication that may result in significant blood loss and potentially death.
One unlikely complication of a PCI procedure is a perforation (hole) in a heart blood vessel. A perforation
can occur when a medical device such as a catheter, guidewire, or stent gets caught in the vessel wall
and pushes through the wall to create a hole that allows blood to escape the vessel. This is a rare,
life-threatening complication that may result in significant blood loss and potentially death.
Current Treatment
Doctors use standard balloon catheters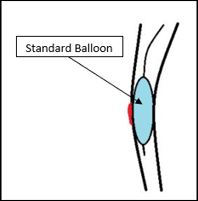 (a medical device commonly
used in PCI procedures) to temporarily control bleeding through a perforation. When the balloon is inflated,
it also blocks blood flow through the vessel, which can lead to a heart attack (death of part of the heart muscle).
As a result, standard balloons should only be inflated for a short time. If the balloon needs to be deflated
before the perforation can be treated, blood loss may continue to occur.
(a medical device commonly
used in PCI procedures) to temporarily control bleeding through a perforation. When the balloon is inflated,
it also blocks blood flow through the vessel, which can lead to a heart attack (death of part of the heart muscle).
As a result, standard balloons should only be inflated for a short time. If the balloon needs to be deflated
before the perforation can be treated, blood loss may continue to occur.
A special stent designed to seal the perforation may be permanently placed in the vessel, or an emergency coronary artery bypass graft (CABG) surgery may be needed. During CABG surgery, veins from the leg or arm or arteries from inside the chest are used to bypass the perforation.
Investigational Device
The Ringer™ catheter is intended to temporarily control bleeding 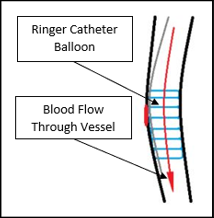 until the perforation can be treated.
The RINGER catheter controls bleeding from a perforation in the same way as a standard balloon catheter.
Unlike a standard balloon that blocks blood flow through the vessel, the RINGER balloon is like a donut
with a hollow center that allows blood to still flow through the vessel. This means that the RINGER
catheter balloon can be inflated for a longer time (up to one hour) while the permanent treatment
method is determined.
until the perforation can be treated.
The RINGER catheter controls bleeding from a perforation in the same way as a standard balloon catheter.
Unlike a standard balloon that blocks blood flow through the vessel, the RINGER balloon is like a donut
with a hollow center that allows blood to still flow through the vessel. This means that the RINGER
catheter balloon can be inflated for a longer time (up to one hour) while the permanent treatment
method is determined.
Clinical Investigation
The Ringer™ catheter is not currently approved by the U.S. Food and Drug Administration (FDA), which means it can only be used by doctors in research investigations like this one.
Patients will undergo a standard, non-experimental PCI procedure to treat an existing heart issue. In the unlikely event of a life-threatening perforation, the doctor will decide if the RINGER catheter can be used to temporarily control bleeding from the perforation until a permanent treatment (such as placement of a covered stent) is determined. Patients will only be enrolled in this clinical investigation if a RINGER catheter is used. The only experimental part of this clinical investigation is the use of the RINGER catheter.
Patients will only be enrolled in this clinical investigation through hospital discharge or 24 hours post-procedure, whichever comes first, and they may choose to withdraw at any time without penalty. The FDA may inspect medical records related to the clinical investigation
The clinical investigation is expected to enroll 30 patients and last approximately 17 months.
Exception from Informed Consent
Written informed consent is usually required before a patient can be enrolled in a clinical investigation. Under special laws for emergency research (called “exception from informed consent”), patients can be enrolled in a clinical investigation without their consent if certain criteria are met.
Exception from informed consent laws apply when:
- • A patient is in a life-threatening emergency, and it is not possible to obtain informed consent
- • There is no reasonable way to identify who would likely meet the criteria for the clinical investigation
- • The clinical investigation could not be carried out without the special laws
This clinical investigation is being conducted under the exception from informed consent laws because a perforation is a life-threatening emergency. Blood loss must be immediately controlled, and there is not enough time for the patient (who is likely under sedation) or his/her legally authorized representative or family member (who is not in the operating room) to give consent.
There is no reasonable way to identify patients ahead of time for this emergency research investigation. Perforations occur in less than 1% of cases and are not specific to any type of PCI procedure. It is estimated that over 14,000 PCI patients would need to be approached to enroll the 30 patients needed for this clinical investigation. This burden on the clinical investigation staff, hospitals, and patients is not practical. Therefore, this investigation would not be possible without use of the exception from informed consent law.
When possible, informed consent will be obtained before a patient is enrolled in the clinical investigation.
Risks and Benefits
There are no guaranteed benefits from use of the RINGER catheter. However, it is possible that use of the RINGER catheter may provide a lower-risk non-surgical means to control blood loss from a perforation compared to currently available devices and treatments. Because the RINGER catheter balloon has a hollow center, doctors can also deliver other medical devices through the center of the balloon during a PCI procedure. This ability to continue treatment after a perforation occurs may eliminate the need for a follow-up procedure.
There are risks associated with all procedures that use medical devices to treat a diseased heart and associated blood vessels. It is believed that the risks associated with use of the Ringer™ Perfusion Balloon Catheter are similar in nature and frequency to the risks of the PCI procedure in general. The frequency and severity of adverse events can vary, and may require additional medical intervention, including surgery.
Clinical Investigation Costs
In this clinical investigation, the only experimental part of the procedure is the use of the RINGER catheter when a life-threatening perforation occurs during a regularly scheduled PCI procedure. The clinical investigation sponsor will pay for the RINGER device and any device-related complications. The clinical investigation sponsor will also pay for certain lab tests that are required at specific times for the clinical investigation.
The patient’s insurance company will still be responsible for the costs of the PCI procedure and all standard-of-care tests, visits, and medicine, as they normally would for a PCI procedure. These costs will include their usual insurance deductibles and co-payments, and all usual insurance rules would apply.
In some cases, insurers may not reimburse claims submitted for standard-of-care medical items, procedures, or treatments if they are performed as part of a research clinical investigation. Prospective patients may want to talk with their insurance company about its payment policy for standard medical care given during a research clinical investigation. If the insurance company does not pay, the patient may be billed for those charges.
Request for Input
The exception from informed consent laws require the sponsor of the clinical investigation to tell the community about this emergency research clinical investigation, listen for concerns, and address those concerns. You are encouraged to provide feedback on this clinical investigation by contacting Vascular Solutions/Teleflex or the participating site using the contact information provided in this brochure.